what does matter have to do with chemistry? how are forces involved?
11.i: States of Matter and Intermolecular Forces
- Page ID
- 41990
Learning Goals
- Identify States of Matter
- Innovate concept of Imf (InterMolecular Forces) or van der Waals forces
- Introduce properties of matter that tin can depend on IMFs
We accept learned how chemical science is the study of matter and how affair transforms from one type of "stuff" into some other. We have been introduced to 4 states of matter and the side by side 2 chapters will await at the condensed phases of matter, the solid and liquid states. We are going to start with a quick review, and so movement into the cohesive forces that concord matter together.
States of Matter:
- Gas Land (Chapter 10)
- Compressible, variable density
- Fluid with negligible resistance to menstruum
- Dispersed state where matter fills unabridged volume of container
- negligible IMFs (InterMolecular Forces)
- Liquid State (Chapter eleven)
- Incompressible, essentially abiding density
- Fluid of measurable viscosity (resistance to flow)
- substantially constant density (varies slightly with temperatue)
- cohesive IMFs hold it together
- Solid Land (Affiliate 12)
- Incompressible, essentially constant density
- Non-Fluid, maintains own shape
- Plasma State (Affiliate 1A.iii.2.4, non thoroughly covered in this class)
- High Energy State (stellar matter)
- Has complimentary electrons and charged particles

Fig. 11.1a: Free energy diagram showing states of water and the phase transitions betwixt these states.
You should already be familiar with the 6 stage transitions described in figure 11.1a
- Melting: The transition from the solid to the liquid phase
- Freezing: The transition from the liquid stage to the solid stage
- Evaporating: The transition from the liquid stage to the gas phase
- Condensing:The transition from the gas phase to the liquid stage
- Sublimation: The transition from the solid phase to the gas phase
- Deposition: The transition from the gas phase to the solid phase
It should be mentioned that the majority of matter in the universe is in a fourth state, the plasma state, which we will not be considering in this chapter. A plasma is a high energy gas with costless moving positive ions and electrons flowing effectually. Stars are in the plasma state and figure 11.1b shows the transitions between all 4 states of affair.
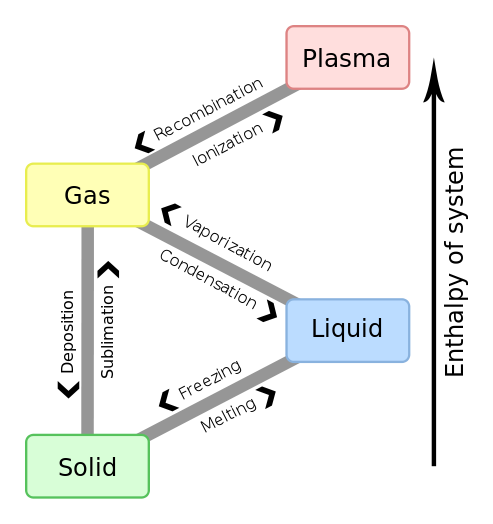
Fig. 11.1b: Four states of matter with transitions between them.
Intermolecular Forces (International monetary fund)
The the term InterMolecular Strength (Imf) literally means the forces between molecules, and as such, is ofttimes a misnomer, as merely speaking, not all matter is composed of molecules. Still this term is used pervasively, and then we will employ it, only first lets compare it to the and so called "intramolecular forces", the forces inside the proverbial "molecule." We typically consider two types of bonds, ionic (which are not molecular) and covalent (which are molecular). And so if I take liquid water, where the detached entity of matter is the HiiO molecule, there are two blazon of bonds, those between H and O of a water molecule (covalent intramolecular) and those betwixt the H of one molecule and the O of some other (intermolecular).
Shortcomings of the term Intermolecular
- We classify bonds between an ion and polar molecule every bit intermolecular, when in reality, the ion need not be a molecule (polyatomic ions are molecules, monatomic ions are not).
- Not all covalently bonded compounds are molecules with discrete formula, like diamond, which is a covalent network, and then even though it is a covalently bonded entity, information technology does non fit the paradigm of a molecule with a unique molecular formula.
With these shortcomings in mind we are going to look at the post-obit types of Intermolecular Forces. It should too be noted that the after types of forces are too called van der Waals forces, which are the short range forces of attraction between atoms or molecules that practice not fit into the model of a covalent or ionic bond.
Types of Intermolecular Forces
- Ion-Dipole Forces (these may non be true Imf, but we will call them IMFs)
- Solutions
- Dipole-Dipole Forces (betwixt two polar molecules)
- Pure substance or solutions
- Hydrogen Bonding (special type of dipole-dipole)
- Pure substance or solutions
- Dipole-Induced Dipole (betwixt polar and nonpolar molecules)
- Solutions
- Instantaneous Dipole-Induced Diploe (betwixt two nonpolar molecules, oftentimes called London Dispersion Forces)
- Solutions or Pure Substances
Please notation that in recognizing what type of intermolecular forces are involved we demand to know if a molecule is polar or non-polar. This requires a review of Lewis Dot Structures, VSEPR Theory, Electronegativety, and Bond Polarity.
Properties of Thing that Depend on IMFs
- boiling point, melting point, stable phases
- enthalpies of phase transitions
- vapor pressure
- solubility and miscibility of different substances
- viscosity
There are many physical backdrop of matter that are strongly influenced by IMFs, and over the next few capacity we will look at many of these. Lets ask a simple question;
Exercise \(\PageIndex{i}\)
At a given temperature, would information technology be easier to vaporize a light molecule or a heavy molecule?
- Answer
-
Yous demand more than information. From our study of gases and the Kinetic Molecular Theory we learned that the boilerplate kinetic free energy of a molecular system is proportional to the absolute temperature, and both molecules accept the same average kinetic energy, and so at first idea, we would predict that the lighter molecule would tend to have the higher velocity (review Graham's Police force of Effusion) and exist easier to vaporize (run into paradigm below).
The problem is we demand to identify the intermolecular forces that attract the molecules to each other, and if the lighter molecule had stronger intermolecular forces, this would be wrong. Expect at the instance of carbon dioxide and h2o (the next question deals with this).
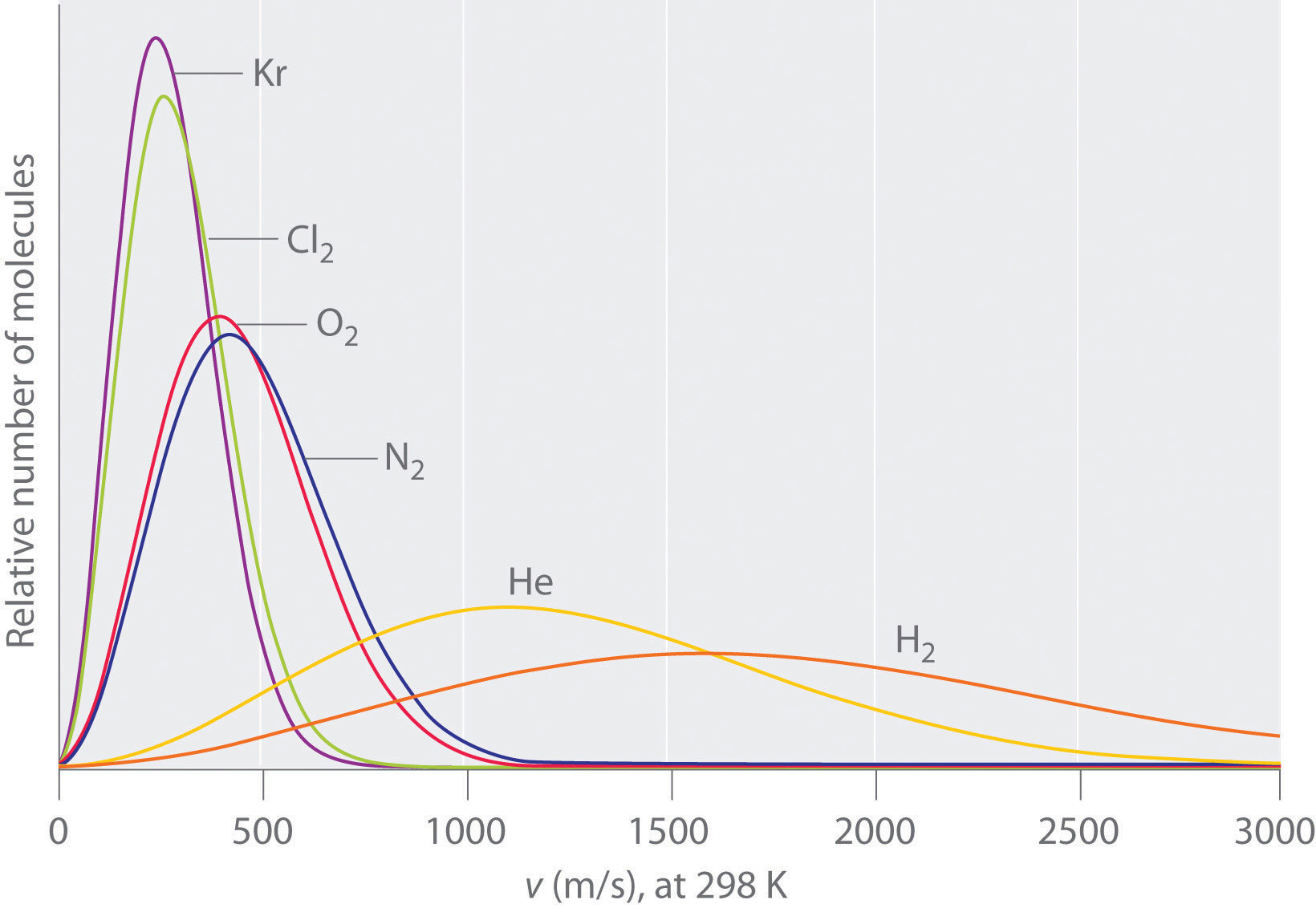
Note: This paradigm is for the velocity distribution of a gas at a given temperature, and not a liquid, but the concept is valid, in that if all other things are equal, lighter molecules tend to motility faster, and gas phase systems move faster than liquid. The problem is, there are often other factors, as indicated in the next question, on why is carbon dioxide a gas at ambient questions while water is a liquid?
Exercise \(\PageIndex{ii}\)
So why at ambient conditions is Carbon Dioxide (the heavier molecule at 44 amu) a gas, but water (the lighter molecule at 18 amu) is a liquid?
- Answer
- That is, shouldn't it be easier to vaporize the lighter molecule than the heavier one? The answer lies inside the Intermolecular Forces, and if you review molecular shapes and polarity you will see that water is a polar molecule while carbon dioxide is non-polar, and this leads to different concrete properties like the boiling and melting points. That is, in this affiliate you will learn that water has stronger intermolecular forces than carbon dioxide, and that is why information technology is a liquid, while carbon dioxide is a gas. Just to reply that question y'all first had to determine the Lewis Dot Structure, then determine the geometry, and and then determine the polarity of the molecule, and so you should have a basic understanding of that pre-requisite cloth earlier proceeding.
Another interesting things to note about molecular properties that result from IMFs is that they are not really properties of a single molecule, only the result of an ensemble or large organization of many molecules, which must be viewed as a whole, and non as private molecules. Simple speaking, an individual molecule can not have a boiling indicate, which is really a office of the interaction betwixt a large number of molecules. So the humid point of water is not a property of a water molecule, merely a agglomeration of water molecules. So, if y'all have a liquid at a given temperature there is a distribution of velocities, some of the molecules take enough kinetic energy to escape the bonny cohesive forces on the surface and enter the vapor phase, while others practise not. And then the vapor pressure in a higher place a liquid is also influenced past intermolecular forces. The point is some properties of a substance, like it's atomic mass or its polarity, are the holding of a single molecule, while others, like the melting point or vapor pressure, are the properties of a huge number (ensemble) of molecules
Source: https://chem.libretexts.org/Courses/University_of_Arkansas_Little_Rock/Chem_1403:_General_Chemistry_2/Text/11:_Intermolecular_Forces_and_Liquids/11.01:_States_of_Matter_and_Intermolecular_Forces
0 Response to "what does matter have to do with chemistry? how are forces involved?"
Post a Comment